Karthik Shekhar and his colleagues raised a few eyebrows as they collected cow and pig eyes from Boston butchers, but those eyes — eventually from 17 separate species, including humans — are providing insights into the evolution of the vertebrate retina and could lead to better animal models for human eye diseases.
The retina is a miniature computer containing diverse types of cells that collectively process visual information before transmitting it to the rest of the brain. In a comparative analysis across animals of the many cell types in the retina — mice alone have 130 types of cells in the retina, as Shekhar’s previous studies have shown — the researchers concluded that most cell types have an ancient evolutionary history. These cell types, distinguished by their differences at the molecular level, give clues to their functions and how they participate in building our visual world.
Their remarkable conservation across species suggests that the retina of the last common ancestor of all mammals, which roamed the earth some 200 million year ago, must have had a complexity rivaling the retina of modern mammals. In fact, there are clear hints that some of these cell types can be traced back more than 400 million years ago to the common ancestors of all vertebrates — that is, mammals, reptiles, birds and jawed fish.
The results will be published Dec. 13 in the journal Nature as part of a 10-paper package reporting the latest results of the BRAIN Initiative Cell Census Network's efforts to create a cell-type atlas of the adult mouse brain. The first author is Joshua Hahn, a chemical and biomolecular engineering graduate student in Shekhar’s group at the University of California, Berkeley. The work was an equal collaboration with the group of Joshua Sanes at Harvard University.
The findings were a surprise, since vertebrate vision varies so widely from species to species. Fish need to see underwater, mice and cats require good night vision, monkeys and humans evolved very sharp daytime eyesight for hunting and foraging. Some animals see vivid colors, while others are content with seeing the world in black and white.
Yet, numerous cell types are shared across a range of vertebrate species, suggesting that the gene expression programs that define these types likely trace back to the common ancestor of jawed vertebrates, the researchers concluded.
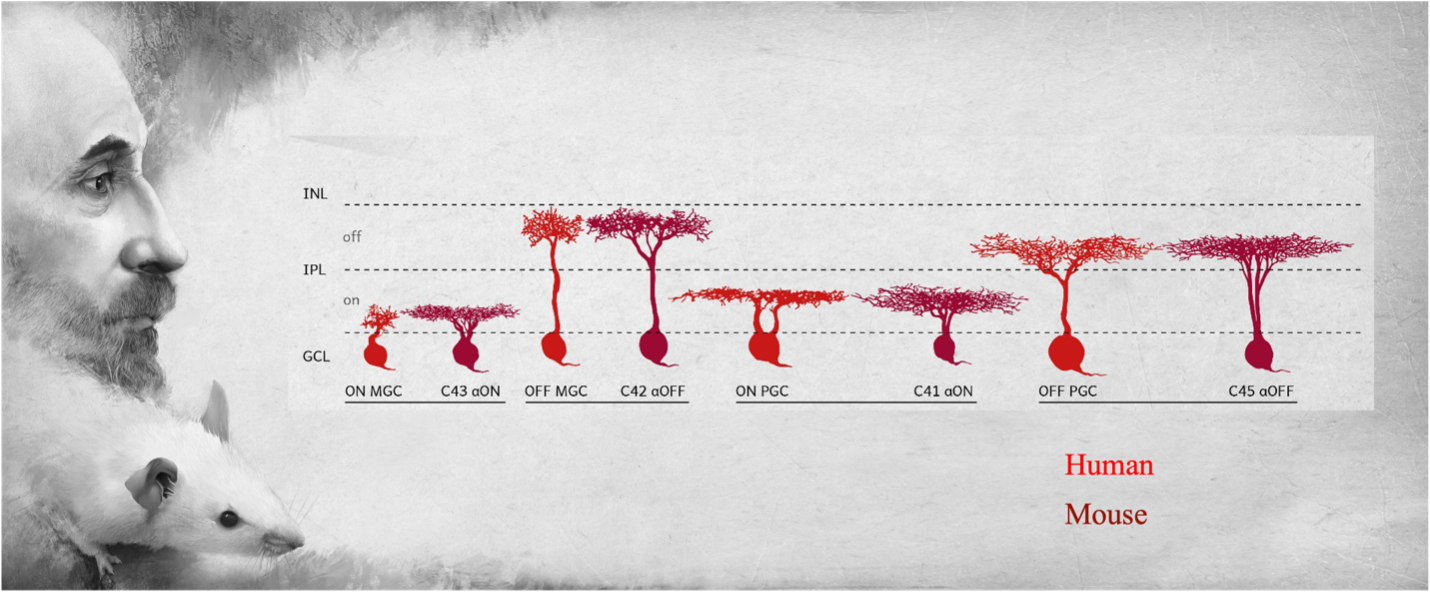
The team found, for example, that one cell type — the “midget” retinal ganglion cell — that is responsible for our ability to see fine detail, is not unique to primates, as it was thought to be. By analyzing large-scale gene expression data using statistical inference approaches, the researchers discovered evolutionary counterparts of midget cells in all other mammals, though these counterparts occurred in much smaller proportions.
“What we are seeing is that something thought to be unique to primates is clearly not unique. It’s a remodeled version of a cell type that is probably very ancient," said Shekhar, a UC Berkeley assistant professor of chemical and biomolecular engineering. "The early vertebrate retina was probably extremely sophisticated, but the parts list has been used, expanded, repurposed or refurbished in all the species that have descended since then."
Coincidentally, one of Shekhar's UC Berkeley colleagues, Teresa Puthussery of the School of Optometry, reported last month in Nature that another cell type thought to have been lost in the human eye — a type of retinal ganglion cell responsible for gaze stabilization — is still there. Puthussery and her colleagues used information from a previous paper co-authored by Shekhar to select molecular markers that helped identify this cell type in primate retinal tissue samples.
The discoveries are, in a sense, not a total surprise, since the eyes of vertebrates have a similar plan: Light is detected by photoreceptors, which relay the signal to bipolar, horizontal and amacrine cells, which in turn connect with retinal ganglion cells, which then relay the results to the brain's visual cortex. Shekhar uses new technologies, in particular single-cell genomics, to assay the molecular composition of thousands to tens of thousands of neurons at once within the visual system, from the retina to the visual cortex.
Because the number of identified retinal cell types varies widely in vertebrates — about 70 in humans, but 130 in mice, based on previous studies by Shekhar and his colleagues — the origins of these diverse cell types were a mystery.
One possibility that emerged from the new research, Shekhar said, is that as the primate brain became more complex, primates began to rely less on signal processing within the eye — which is key to reflexive actions, such as reacting to an approaching predator — and more on analysis within the visual cortex. Hence the apparent decrease in molecularly distinct cell types in the human eye.
"Our study is saying that the human retina may have evolved to trade cell types that perform sophisticated visual computations for cell types that basically just transmit a relatively unprocessed image of the visual world with the brain so that we can do a lot more sophisticated things with that," Shekhar said. "We are giving up speed for finesse."
The team's new detailed map of cell types in a variety of vertebrate retinas could aid research on human eye disease. Shekhar’s group is also studying molecular hallmarks of glaucoma, the leading cause of irreversible blindness in the world and, in the U.S., the second most common cause of blindness after macular degeneration.
Yet, while mice are a favorite model animal for studying glaucoma, they have very few of the midget retinal ganglion cell counterparts. These cell types make up only 2% to 4% of all ganglion cells in mice, whereas 90% of retinal ganglion cells are midget cells in humans.
"This work is clinically important because, ultimately, the midget cells are probably what we should care about the most in human glaucoma," Shekhar said. "Knowing their counterparts in the mouse will hopefully help us design and interpret these glaucoma mouse models a little better."
Single-cell transcriptomics
Shekhar and Sanes have, for the past eight years, been applying single-cell genomic approaches to profile the mRNA molecules in cells to categorize them according to their gene expression fingerprints. That technique has gradually helped identify more and more distinct cell types within the retina, many of them through studies that Shekhar initiated while a postdoctoral fellow with Aviv Regev, one of the pioneers of single-cell genomics, at the Broad Institute. It was in her lab that Shekhar began working with Sanes, a renowned retinal neurobiologist who became Shekhar’s co-advisor and collaborator.
In the current study, they wanted to expand their single-cell transcriptomic approach to other species to understand how retinal cell types have changed through evolution. They gathered, in all, eyes from 17 species: human, two monkeys (macaque and marmoset), four rodents (three species of mice and one ground squirrel), three ungulates (cow, sheep and pig), tree shrew, opossum, ferret, chicken, lizard, zebrafish and lamprey.
With Sanes' team at Harvard conducting the transcriptomic experiments and Shekhar's team at UC Berkeley conducting the computational analysis, many new cell types were identified in each of the species. They then mapped this variety to a smaller set of "orthotypes" — cell types that have likely descended from the same ancestral cell type in early vertebrates.
For bipolar cells, which are a class of neurons that lie between the photoreceptors and retinal ganglion cells, they found 14 distinct orthotypes. Most extant species contain 13 to 16 bipolar types, suggesting that these types have evolved little. In contrast, they found 21 orthotypes of retinal ganglion cells, which exhibit greater variation among species. Studies thus far have identified more than 40 distinct types in mice and about 20 different types in humans.
Interestingly, the pronounced evolutionary divergence among types of retinal ganglion cells, as compared to other retinal classes, suggests that natural selection acts more strongly on diversifying neuron types that transmit information from the retina to the rest of the brain.
They also found that numerous transcription factors, which have been implicated in retinal cell type specification in mice, are highly conserved, suggesting that the molecular steps leading to the development of the retina might be evolutionarily conserved, as well.
Based on the new work, Shekhar is refocusing his glaucoma research on the analogs of midget cells, called alpha cells, in mice.
The work was supported primarily by the National Institutes of Health (K99EY033457, R00EY028625, R21EY028633, U01MH105960, T32GM007103), the Chan-Zuckerberg Initiative (CZF-2019-002459) and the Glaucoma Research Foundation (CFC4). Shekhar also acknowledges support from the Hellman Fellows Program.
This story was first published by Berkeley News.









